Ukuminyana Kwebhethri
Okuqukethwe
Ukuminyana kwe-electrolyte ebhethri kuyipharamitha ebaluleke kakhulu kuwo wonke amabhethri e-asidi, futhi noma yimuphi umshisekeli wemoto kufanele azi: kufanele kube yini ukuminyana, ukuthi ungayihlola kanjani, futhi okubaluleke kakhulu, indlela yokukhulisa kahle ukuminyana kwebhethri (okuqondile). amandla adonsela phansi e-asidi) ethini ngalinye elinamapuleti omthofu agcwele isisombululo se-H2SO4.
Ukuhlola ukuminyana kungenye yamaphuzu enqubweni yokugcinwa kwebhethri, okuhlanganisa nokuhlola izinga le-electrolyte nokulinganisa i-voltage yebhethri. kumabhethri omthofu ukuminyana kukalwa ngo-g/cm3. It ngokulingana nokugxila kwesixazululo, futhi kuncike ngokuphambene nezinga lokushisa uketshezi (lapho izinga lokushisa liphakeme, liyancipha ukuminyana).
Ngobuningi be-electrolyte, ungakwazi ukunquma isimo sebhethri. Ukuze uma ibhethri lingashayi, khona-ke kufanele uhlole isimo soketshezi lwayo kuwo wonke amabhange.
Ukuminyana kwe-electrolyte kuthinta umthamo webhethri nempilo yayo yesevisi.
Ihlolwe nge-densimeter (hydrometer) ekushiseni kuka +25 ° С. Uma izinga lokushisa lihluka kulokho okudingekayo, ukufundwa kuyalungiswa njengoba kukhonjisiwe etafuleni.
Ngakho-ke, sithole kancane ukuthi kuyini, nokuthi yini okudingeka ihlolwe njalo. Futhi yiziphi izinombolo okufanele ugxile kuzo, kungakanani okuhle nokubi kangakanani, kufanele kube yini ukuminyana kwe-electrolyte yebhethri?
Yikuphi ukuminyana okufanele kube kubhethri
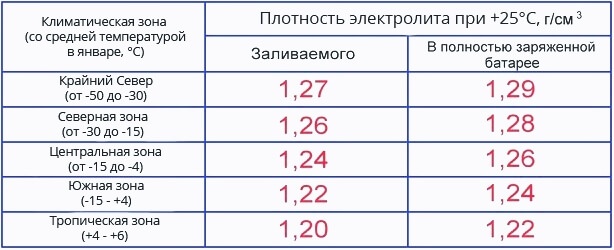
Ukugcina ukuminyana kwe-electrolyte kubaluleke kakhulu ebhethrini futhi kufanelekile ukwazi ukuthi amanani adingekayo ancike endaweni yesimo sezulu. Ngakho-ke, ukuminyana kwebhethri kufanele kusethwe ngokusekelwe kwinhlanganisela yezidingo nezimo zokusebenza. Ngokwesibonelo, esimweni sezulu esishisayo, ukuminyana kwe-electrolyte kufanele ibe sezingeni 1,25-1,27 g/cm3 ±0,01 g/cm3. Ezindaweni ezibandayo, lapho ubusika bufinyelela ku--30 degrees, 0,01 g / cm3 ngaphezulu, nasezindaweni ezishisayo ezishisayo - 0,01 g/cm3 ngaphansi. Kulezo zifunda lapho ubusika bunzima kakhulu (kufika ku -50 ° C), ukuze ibhethri lingaqhwa, kufanele ukwandisa ukuminyana kusuka ku-1,27 kuya ku-1,29 g/cm3.
Abanikazi bezimoto abaningi bayazibuza: “Kufanele kube yini ukuminyana kwe-electrolyte ebhethrini ebusika, futhi kufanele kube yini ehlobo, noma akukho mehluko, futhi kufanele izinkomba zigcinwe zisezingeni elifanayo unyaka wonke?” Ngakho-ke, sizobhekana nendaba ngokuningiliziwe, futhi kuzosiza ukuyikhiqiza, Ithebula lebhethri le-electrolyte density ihlukaniswe yaba izindawo zezulu.
udinga futhi ukukhumbula ukuthi, ngokuvamile, ibhethri, ukuba ngemoto, engakhokhiswa ngaphezu kuka-80-90% umthamo wayo okuzisholo, ngakho ukuminyana electrolyte kuyoba ngaphansi kancane kunalokho lapho icala ngokugcwele. Ngakho-ke, inani elidingekayo likhethwa liphakeme kancane, kusukela kulelo elikhonjiswe etafuleni lokuminyana, ukuze lapho izinga lokushisa lomoya lehla lifika ezingeni eliphakeme, ibhethri liqinisekisiwe ukuthi lizohlala lisebenza futhi lingaqandi ebusika. Kodwa, mayelana nenkathi yasehlobo, ukuminyana okwandayo kungasongela ukubila.
Ithebula lokuminyana kwe-electrolyte yebhethri
Ithebula lokuminyana lihlanganiswa ngokuhambisana nezinga lokushisa elivamile lanyanga zonke ngenyanga kaJanuwari, ukuze izindawo zezulu ezinomoya obandayo zifike ku--30 ° C futhi ezilinganiselwe ezinamazinga okushisa angekho ngaphansi kuka -15 azidingi ukwehla noma ukwanda kokuhlushwa kwe-asidi. . Unyaka wonke (ebusika nasehlobo) ukuminyana kwe-electrolyte ebhethrini akufanele kushintshwe, kodwa hlola kuphela futhi qiniseka ukuthi ayisuki enanini legama, kodwa ezindaweni ezibandayo kakhulu, lapho izinga lokushisa ngokuvamile lingaphansi kwama-degrees angu-30 (enyameni kuze kufike ku-50), ukulungiswa kuvunyelwe.
Ukuminyana kwe-electrolyte ebhethrini ebusika
Ukuminyana kwe-electrolyte ebhethrini ebusika kufanele kube ngu-1,27 (ezifundeni ezinamazinga okushisa asebusika angaphansi -35, hhayi ngaphansi kuka-1.28 g/cm3). Uma inani liphansi, khona-ke lokhu kuholela ekwehleni kwamandla e-electromotive kanye nokuqala okunzima kwenjini evuthayo yangaphakathi esimweni sezulu esibandayo, kuze kube yiqhwa le-electrolyte.
Lapho ukuminyana kwebhethri kwehliswa ebusika, akufanele ugijimele ngokushesha isixazululo sokulungisa ukuze usikhuphule, kungcono kakhulu ukunakekela enye into - ukushaja kwebhethri kwekhwalithi ephezulu usebenzisa ishaja.
Uhambo lwehora lesigamu ukusuka ekhaya ukuya emsebenzini nangemuva aluvumeli i-electrolyte ukuthi ifudumale, ngakho-ke, izoshajwa kahle, ngoba ibhethri ithatha icala kuphela ngemva kokufudumala. Ngakho-ke i-rarefaction iyanda usuku nosuku, futhi ngenxa yalokho, ukuminyana nakho kuyancipha.
Kubhethri elisha nelisebenzisekayo, isikhawu esivamile sokushintsha ukuminyana kwe-electrolyte (ukukhishwa okugcwele - ukushajwa okugcwele) ngu-0,15-0,16 g/cm³.
Khumbula ukuthi ukusebenza kwebhethri elilahliwe emazingeni okushisa angaphansi kukaziro kuholela emakhazeni ama-electrolyte kanye nokucekelwa phansi kwamapuleti omthofu!
Ngokwethebula lokuncika kwendawo yokuqhwa ye-electrolyte ekumineni kwayo, ungathola umkhawulo wokususa wekholomu yethemometha lapho iqhwa kwakheka khona ebhethrini lakho.
g/cm³ | 1,10 | 1,11 | 1,12 | 1,13 | 1,14 | 1,15 | 1,16 | 1,17 | 1,18 | 1,19 | 1,20 | 1,21 | 1,22 | 1,23 | 1,24 | 1,25 | 1,28 |
° C | -8 | -9 | -10 | -12 | -14 | -16 | -18 | -20 | -22 | -25 | -28 | -34 | -40 | -45 | -50 | -54 | -74 |
Njengoba ubona, uma ishajwe kufika ku-100%, ibhethri izoba yiqhwa ku -70 °C. Ngokushaja okungu-40%, iqhwa kakade ku-25 ° C. U-10% ngeke nje ukwenze kungenzeki ukuqalisa injini evuthayo yangaphakathi ngosuku oluyisithwathwa, kodwa izobanda ngokuphelele ku-10 degree frost.
Uma ukuminyana kwe-electrolyte kungaziwa, izinga lokuphuma kwebhethri lihlolwa ngepulaki yokulayisha. Umehluko kagesi kumaseli ebhethri elilodwa akufanele udlule u-0,2V.
Ukufundwa kwe-voltmeter yepulaki yokulayisha, B | Idigri yokukhishwa kwebhethri, % |
1,8-1,7 | 0 |
1,7-1,6 | 25 |
1,6-1,5 | 50 |
1,5-1,4 | 75 |
1,4-1,3 | 100 |
Uma ibhethri lishajwa ngaphezu kuka-50% ebusika nangaphezulu kuka-25% ehlobo, kufanele lishajwe kabusha.
Ukuminyana kwe-electrolyte ebhethrini ehlobo
Ehlobo, ibhethri linenkinga yokuphelelwa amanzi emzimbeni., ngakho-ke, kunikezwe ukuthi ukuminyana okwandayo kunomphumela omubi kumapuleti okuhola, kungcono uma kunjalo 0,02 g/cm³ ngaphansi kwevelu edingekayo (ikakhulukazi ezifundeni eziseningizimu).
Ehlobo, izinga lokushisa ngaphansi kwe-hood, lapho ibhethri livame ukutholakala khona, likhuphuka kakhulu. Izimo ezinjalo zinomthelela ekuhwamukeni kwamanzi kusuka ku-asidi kanye nomsebenzi wezinqubo ze-electrochemical ebhethrini, okuhlinzeka ngokukhipha okuphezulu kwamanje ngisho nasezingeni eliphansi elivumelekile le-electrolyte (1,22 g/cm3 endaweni efudumele yesimo sezulu). Ukuze, lapho izinga le-electrolyte lehla kancane kancane, khona-ke ukuminyana kwawo kuyanda, okusheshisa izinqubo zokubhujiswa kokugqwala kwama-electrode. Yingakho kubaluleke kakhulu ukulawula izinga le-liquid ebhethri futhi, lapho lehla, engeza amanzi a-distilled, futhi uma lokhu kungenziwanga, ukushaja ngokweqile kanye ne-sulfation kusongela.
Uma ibhethri likhishwa ngenxa yokunganaki komshayeli noma ezinye izizathu, kufanele uzame ukulibuyisela esimweni salo sokusebenza usebenzisa ishaja. Kodwa ngaphambi kokushaja ibhethri, babheka izinga futhi, uma kunesidingo, bagcwalise ngamanzi a-distilled, angahwamuka ngesikhathi sokusebenza.
Ngemva kwesikhathi esithile, ukuminyana kwe-electrolyte ebhethrini, ngenxa yokuhlanjululwa kwayo njalo nge-distillate, kuncipha futhi kwehla ngaphansi kwenani elidingekayo. Khona-ke ukusebenza kwebhethri kuba yinto engenakwenzeka, ngakho-ke kuyadingeka ukwandisa ukuminyana kwe-electrolyte ebhethri. Kodwa ukuze uthole ukuthi kungakanani ukwanda, udinga ukwazi ukuthi ungabheka kanjani lokhu kuminyana.
Ungahlola kanjani ukuminyana kwebhethri
Ukuqinisekisa ukusebenza kahle kwebhethri, ubukhulu be-electrolyte iyaqhubeka hlola njalo 15-20 ayizinkulungwane km gijima. Isilinganiso sokuminyana ebhethrini senziwa kusetshenziswa idivayisi efana ne-densimeter. Idivayisi yale divayisi iqukethe ithubhu yengilazi, ngaphakathi okuyi-hydrometer, futhi ekugcineni kukhona ithiphu yenjoloba ngakolunye uhlangothi kanye nepheya ngakolunye. ukuze uhlole, uzodinga: vula ukhokho webhethri can, ucwiliswe esixazululweni, bese udweba inani elincane le-electrolyte ngepheya. I-hydrometer entantayo enesilinganiso izokhombisa lonke ulwazi oludingekayo. Sizocabangela ngokuningiliziwe indlela yokuhlola kahle ukuminyana kwebhethri kancane kancane, njengoba kukhona uhlobo olunjalo lwebhethri njengokuthi alunakugcinwa, futhi inqubo ihluke kubo - ngeke udinge noma iyiphi idivayisi.
Inkomba yokuminyana kubhethri elingalungiswa
Ukuminyana kwebhethri elingalungiswa kuboniswa yinkomba yombala efasiteleni elikhethekile. Inkomba eluhlaza kufakaza lokho Konke kulungile (degree of charge ngaphakathi 65 - 100%) uma ukuminyana wehlile futhi ukushajwa kabusha kuyadingeka, bese inkomba izokwenza omnyama. Lapho iwindi livela isibani esimhlophe noma esibomvu, khona-ke udinga ukugcwalisa ngokushesha ngamanzi a-distilled. Kodwa, ngendlela, ulwazi oluqondile mayelana nencazelo yombala othile efasiteleni lukusitika sebhethri.
Manje siqhubeka nokuqonda kabanzi ukuthi singahlola kanjani ukuminyana kwe-electrolyte yebhethri evamile ye-asidi ekhaya.
Ihlola ukuminyana kwe-electrolyte ebhethrini
Ngakho-ke, ukuze sikwazi ukuhlola kahle ukuminyana kwe-electrolyte ebhethri, okokuqala sihlola izinga futhi, uma kunesidingo, silungise. Bese sishaja ibhethri bese kuphela siqhubekela ekuhlolweni, kodwa hhayi ngokushesha, kodwa ngemva kwamahora ambalwa okuphumula, kusukela ngokushesha ngemva kokushaja noma ukungeza amanzi kuzoba nedatha engalungile.
Kufanele kukhunjulwe ukuthi ukuminyana kuncike ngokuqondile ekushiseni komoya, ngakho-ke bheka ithebula lokulungisa okukhulunywe ngalo ngenhla. Ngemva kokuthatha uketshezi ebhethrini, bamba idivayisi ezingeni lamehlo - i-hydrometer kufanele iphumule, intante oketshezini, ngaphandle kokuthinta izindonga. Ukulinganisa kwenziwa engxenyeni ngayinye, futhi zonke izinkomba ziyarekhodwa.
Ithebula lokunquma ukushajwa kwebhethri ngokuminyana kwe-electrolyte.
Temperature | Ukushaja | ||
ngo-100% | ngo-70% | Ikhishiwe | |
ngaphezu +25 | 1,21 - 1,23 | 1,17 - 1,19 | 1,05 - 1,07 |
ngaphansi +25 | 1,27 - 1,29 | 1,23 - 1,25 | 1,11 - 1,13 |
Ukuminyana uma kuqhathaniswa ne-voltage ngokuya ngeshaji
Ukuminyana okuncishiswe kakhulu kwelinye lamaseli kubonisa ukuba khona kwamaphutha kuwo (okungukuthi, isifunda esifushane phakathi kwamapuleti). Kodwa uma iphansi kuwo wonke amaseli, khona-ke lokhu kubonisa ukukhishwa okujulile, i-sulfation, noma ukumane kuphelelwe yisikhathi. Ukuhlola ukuminyana, kuhlanganiswe nokulinganisa i-voltage ngaphansi komthwalo nangaphandle, kuzonquma imbangela yangempela yokuphuka.
Uma udinga ukuhlola ukuminyana kwe-electrolyte ukuze unqume izinga lokushaja kwebhethri, ungakwenza lokhu ngaphandle kokukhipha ibhethri ngaphansi kwe-hood yemoto; uzodinga idivayisi ngokwayo, i-multimeter (yokulinganisa i-voltage) kanye netafula lesilinganiso sedatha yokulinganisa.
Shaja amaphesenti | Ukuminyana kwe-Electrolyte g/cm³ (**) | Amandla ebhethri V (***) |
100% | 1,28 | 12,7 |
80% | 1,245 | 12,5 |
60% | 1,21 | 12,3 |
40% | 1,175 | 12,1 |
20% | 1,14 | 11,9 |
0% | 1,10 | 11,7 |
Uma kunesidingo, ukulungiswa kokuminyana kuyenziwa. Kuzodingeka ukuthi ukhethe ivolumu ethile ye-electrolyte ebhethri bese wengeza ukulungisa (1,4 g / cm3) noma amanzi acwecwe, kulandele imizuzu engu-30 yokushaja okulinganiselwe okwamanje kanye nokuchayeka amahora ambalwa ukulinganisa ukuminyana kuwo wonke amagumbi. Ngakho-ke, sizokhuluma ngokuqhubekayo mayelana nendlela yokukhulisa kahle ukuminyana ebhethri.
Ungakhuphula kanjani ukuminyana ebhethrini
Kudingekile ukuphakamisa ukuminyana lapho kudingekile ukulungisa ngokuphindaphindiwe izinga nge-distillate noma akwanele ekusebenzeni kwasebusika kwebhethri, kanye nangemva kokuphindaphinda kabusha okuphindaphindiwe kwesikhathi eside. Uphawu lwesidingo senqubo enjalo kuzoba ukuncishiswa kwesikhawu sokushaja / sokukhipha. Ngaphezu kokushaja ibhethri ngendlela efanele nangokugcwele, kunezindlela ezimbalwa zokwandisa ukuminyana:
- engeza i-electrolyte egxilile (okuthiwa ukulungisa);
- engeza i-asidi.

Ungahlola kanjani kahle futhi ukwandise ukuminyana ebhethrini.
Ukwandisa futhi ulungise ukuminyana kwe-electrolyte ebhethrini, uzodinga:
1) i-hydrometer;
2) inkomishi yokulinganisa;
3) isitsha sokuhlanjululwa kwe-electrolyte entsha;
4) eliphakathi kwe enema;
5) i-electrolyte yokulungisa noma i-asidi;
6) amanzi distilled.
Umongo wenqubo umi kanje:
- Inani elincane le-electrolyte lithathwa ebhange lebhethri.
- Esikhundleni sesamba esifanayo, sengeza i-electrolyte yokulungisa, uma kudingekile ukwandisa ukuminyana, noma amanzi acwecwe (nge-density ye-1,00 g / cm3), uma, ngokuphambene nalokho, ukwehla kwawo kuyadingeka;
- khona-ke ibhethri kufanele ifakwe ekushajweni kabusha, ukuze uyishaje ngesilinganiso samanje isigamu sehora - lokhu kuzovumela uketshezi ukuba luxube;
- Ngemva kokunqamula ibhethri kudivayisi, kuzodingeka futhi ukulinda okungenani ihora/amabili, ukuze ukuminyana kuwo wonke amabhange kuphume, izinga lokushisa lehle kanye nawo wonke ama-bubbles egesi aphume ukuze kuqedwe iphutha ekulawuleni. ukulinganisa;
- Phinda uhlole ukuminyana kwe-electrolyte futhi, uma kunesidingo, phinda inqubo yokukhetha nokwengeza uketshezi oludingekayo (futhi ukwandisa noma ukunciphisa), unciphise isinyathelo sokuhlanjululwa, bese ulinganisa futhi.
ukuze uqonde ukuthi ungakhuphula kanjani ukuminyana ebhethri, noma mhlawumbe ngokuphambene nalokho - udinga ukuncipha kwegumbi lebhethri elilinganiswe ngokukhethekile, kuyinto efiselekayo ukwazi ukuthi iyini ivolumu yokulinganisa kuyo ngamasentimitha angama-cubic. Isibonelo, umthamo we-electrolyte kwelinye ibhange lebhethri yomshini we-55 Ah, i-6ST-55 ingu-633 cm3, kanti i-6ST-45 ingu-500 cm3. Ingxenye yokwakheka kwe-electrolyte cishe kanjena: i-sulfuric acid (40%); amanzi distilled (60%). Ithebula elingezansi lizokusiza ukuthi ufinyelele ukuminyana kwe-electrolyte edingekayo ebhethrini:
i-electrolyte density formula
Sicela uqaphele ukuthi leli thebula lihlinzekela ukusetshenziswa kwe-electrolyte yokulungisa enesisindo esingu-1,40 g/cm³ kuphela, futhi uma uketshezi lunobuningi obuhlukile, kufanele kusetshenziswe ifomula eyengeziwe.
Kulabo abathola izibalo ezinjalo ziyinkimbinkimbi kakhulu, konke kungenziwa kalula ngokusebenzisa indlela yesigaba segolide:
Sipompa uketshezi oluningi ethini lebhethri bese silithela enkomishini yokulinganisa ukuze sithole umthamo, bese sengeza ingxenye yalelo nani le-electrolyte, siyinyakazise ukuze ixube. Uma futhi ukude nenani elidingekayo, bese wengeza ingxenye yesine yevolumu ekhishwe ngaphambilini nge-electrolyte. Ngakho-ke kufanele ifakwe phezulu, isikhathi ngasinye ihlukanise inani, kuze kufike umgomo.
Ungakhuphula kanjani ukuminyana ku-accumulator uma iwela ngaphansi kwe-1.18
Uma ukuminyana kwe-electrolyte kungaphansi kuka-1,18 g/cm3, asikwazi ukwenza nge-electrolyte eyodwa, kuzodingeka sengeze i-asidi (1,8 g/cm3). Inqubo yenziwa ngokuvumelana nesimiso esifanayo nasesimweni sokwengeza i-electrolyte, kuphela sithatha isinyathelo esincane sokuhlanjululwa, ngoba ukuminyana kuphezulu kakhulu futhi ungakwazi ukweqa uphawu olufunayo kakade kusukela ekuhlanjululweni kokuqala.
Isilinganiso sempilo yesevisi yamabhethri anamuhla, ngaphansi kwemithetho yokusebenza (ukuvimbela ukukhishwa okujulile nokushaja ngokweqile, kuhlanganise nephutha lomlawuli we-voltage), iminyaka engu-4-5. Ngakho-ke akunangqondo ukwenza ama-manipulations, njengokuthi: ukubhoboza icala, ukuliphendulela ukukhipha lonke uketshezi futhi ubeke esikhundleni ngokuphelele - lokhu "umdlalo" ophelele - uma amapuleti ewile, akukho lutho olungenziwa. Qaphela ukushaja, hlola ukuminyana ngesikhathi, gcina kahle ibhethri yemoto futhi uzonikezwa imigqa ephezulu yomsebenzi wayo.

