Iviki elisha nebhethri elisha: I-Na-ion (i-sodium-ion), efanayo ngamapharamitha ku-Li-ion, kodwa izikhathi eziningi ishibhile
Abacwaningi baseWashington State University (WSU) benze ibhethri "elinosawoti owengeziwe" esebenzisa i-sodium esikhundleni se-lithium. I-sodium (Na) ingeyeqembu lezinsimbi ze-alkali, inamakhemikhali afanayo, ngakho amaseli asekelwe kuyo anethuba lokuncintisana ne-Li-ion. Okungenani kwezinye izinhlelo zokusebenza.
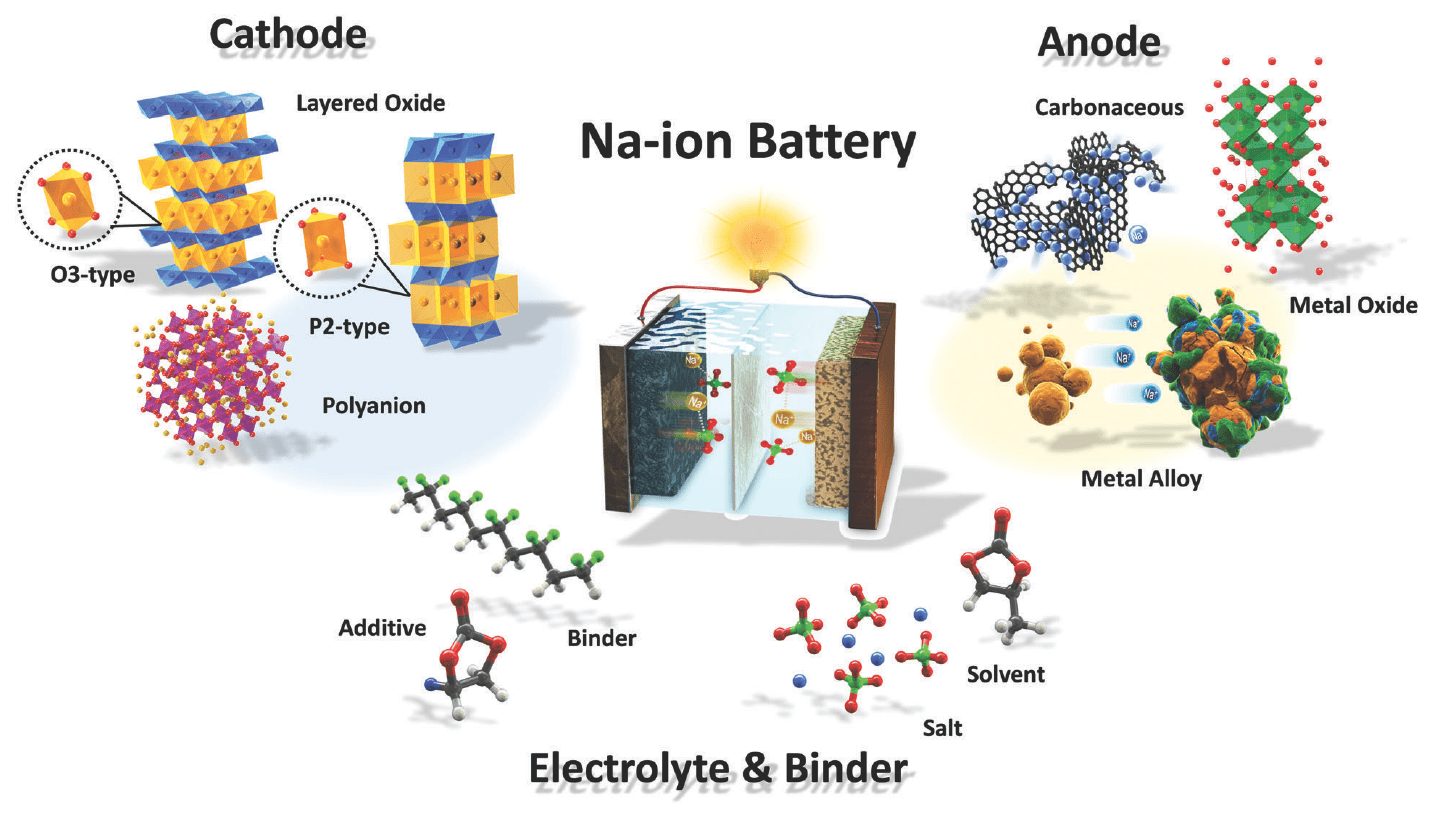
Amabhethri e-Na-ion: ashibhe kakhulu, angaphansi kancane kune-lithium-ion, esigabeni socwaningo
I-sodium ingenye yezakhi ezimbili ku-sodium chloride (NaCl) i-sodium chloride. Ngokungafani ne-lithium, itholakala ngobuningi kuma-deposits (usawoti wamatshe) nasezilwandle nasezilwandle. Ngenxa yalokho, amaseli e-Na-ion angaba ishibhile izikhathi eziningi kunamaseli e-lithium-ion, futhi ngendlela, kufanele aklanywe kusetshenziswa izinto ezifanayo nezakhiwo njengamaseli e-lithium-ion.
Umsebenzi kumaseli e-Na-ion wenziwa eminyakeni engaba ngu-50-40 edlule, kodwa wabuye wanqanyulwa. I-sodium ion inkulu kune-lithium ion, ngakho-ke izakhi zinenkinga yokugcina ukushaja okufanele. Ukwakheka kwegraphite - enkulu ngokwanele i-lithium ion - kuvele kuminyene kakhulu ku-sodium.
Ucwaningo luvuselelekile eminyakeni embalwa edlule njengoba isidingo sezingxenye zikagesi ezisebenziseka kabusha senyuke kakhulu. Ososayensi be-WSU benze ibhethri le-sodium-ion okufanele ligcine inani lamandla afana nalawo angagcinwa ebhethrini elifanayo le-lithium-ion. Ngaphezu kwalokho, ibhethri lithathe umjikelezo wokushaja ongu-1 futhi ligcine ngaphezu kwamaphesenti angu-000 womthamo walo wangempela (owokuqala).
Zombili lezi zinhlaka zibhekwa "njengezinhle" emhlabeni wamabhethri e-lithium-ion. Kodwa-ke, kuma-elementi ane-sodium ion, ukuhambisana nezimo kube nzima ngenxa yokukhula kwamakristalu e-sodium ku-cathode. Ngakho-ke, kwanqunywa ukuthi kusetshenziswe ungqimba oluvikelayo lwe-oxide yensimbi kanye ne-electrolyte ene-sodium ion encibilikile, eyaqinisa isakhiwo. Iphumelele.
I-downside yeseli ye-Na-ion ukuminyana kwayo okuphansi kwamandla, okuqondakalayo uma ucabangela ubukhulu be-lithium ne-athomu ye-sodium. Nokho, nakuba le nkinga ingaba yinkinga emotweni kagesi, ayithinti ngokuphelele ukugcinwa kwamandla. Ngisho noma i-Na-ion ithatha indawo ephindwe kabili njenge-lithium-ion, inani layo eliphindwe kabili noma kathathu liphansi lizokwenza ukukhetha kube sobala.
Yilokhu kuphela kokuqala eminyakeni embalwa ...
Lokhu kungase kube nentshisekelo kuwe:
