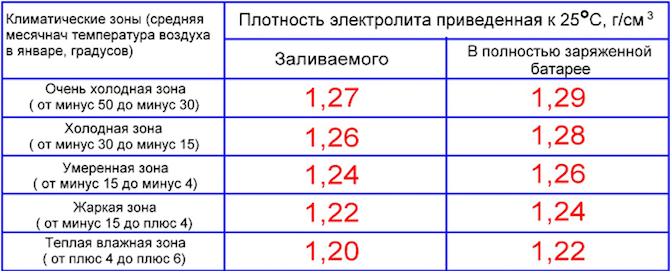
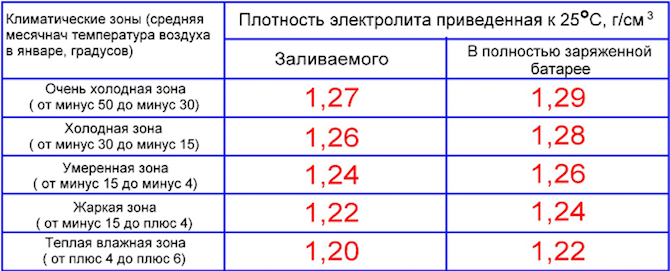
Ukuminyana kwe-electrolyte ebhethrini - ebusika nasehlobo: itafula
Amabhethri amaningi athengiswa eRussia asebenza kancane. Lokhu kusho ukuthi umnikazi angakwazi ukukhulula ama-plugs, ahlole izinga nokushuba kwe-electrolyte futhi, uma kunesidingo, engeza amanzi a-distilled ngaphakathi. Wonke amabhethri e-asidi ngokuvamile akhokhiswa amaphesenti angu-80 lapho eqala ukudayiswa. Lapho uthenga, qiniseka ukuthi umdayisi wenza isheke langaphambi kokudayiswa, elinye lamaphuzu okuwukuhlola ukuminyana kwe-electrolyte kwelinye lamathini.
Esihlokweni sanamuhla ku-portal yethu ye-Vodi.su, sizocubungula umqondo we-electrolyte density: ukuthi iyini, ukuthi kufanele ibe njani ebusika nasehlobo, indlela yokuyandisa.
Emabhethri e-asidi, isisombululo se-H2SO4, okungukuthi, i-sulfuric acid, sisetshenziswa njenge-electrolyte. Ubuningi buhlobene ngokuqondile nephesenti yesisombululo - isibabule esiningi, siphezulu. Esinye isici esibalulekile izinga lokushisa kwe-electrolyte ngokwayo kanye nomoya ozungezile. Ebusika, ukuminyana kufanele kube phezulu kunasehlobo. Uma iwela ezingeni elibucayi, khona-ke i-electrolyte izomane iqhwa nayo yonke imiphumela elandelayo.

Le nkomba ikalwa ngamagremu cubic centimeter ngayinye - g / cm3. Kukalwa kusetshenziswa umshini olula we-hydrometer, okuyiflask yengilazi enepheya ekugcineni kanye nentanta enesikalo phakathi. Lapho uthenga ibhethri elisha, umdayisi unesibopho sokukala ukuminyana, kufanele kube, kuye ngokuthi indawo yendawo kanye nesimo sezulu, 1,20-1,28 g / cm3. Umehluko phakathi kwamabhange awukho ngaphezu kuka-0,01 g/cm3. Uma umehluko mkhulu, lokhu kubonisa ukujikeleza okufushane okungenzeka kwelinye lamaseli. Uma ukuminyana kuphansi ngokulinganayo kuwo wonke amabhange, lokhu kubonisa kokubili ukukhishwa okuphelele kwebhethri kanye ne-sulfation yamapuleti.
Ngaphezu kokulinganisa ukuminyana, umdayisi kufanele futhi ahlole ukuthi ibhethri liwubamba kanjani umthwalo. Ukuze wenze lokhu, sebenzisa imfoloko yokulayisha. Okufanelekile, i-voltage kufanele yehle isuka ku-12 kuya ku-XNUMX volts futhi ihlale kuleli phawu isikhashana. Uma iwa ngokushesha, futhi i-electrolyte kwelinye lamathini ibilisa futhi ikhiphe umusi, kufanele wenqabe ukuthenga leli bhethri.
Ukuminyana ebusika nasehlobo
Ngemininingwane eyengeziwe, le pharamitha yemodeli yebhethri yakho ethile kufanele ifundwe ekhadini lewaranti. Kudalwe amatafula akhethekile wamazinga okushisa ahlukahlukene lapho i-electrolyte ingaba yiqhwa. Ngakho, ekumineni okungu-1,09 g/cm3, ukuqanda kwenzeka ku -7°C. Ezimweni ezisenyakatho, ukuminyana kufanele kudlule i-1,28-1,29 g / cm3, ngoba ngalesi sikhombi, izinga lokushisa elibandayo lingu-66 ° C.
Ukuminyana kuvame ukuboniswa ekushiseni komoya okungu +25°C. Kufanele kube ngeyebhethri egcwele:
- 1,29 g/cm3 - emazingeni okushisa asukela ku -30 kuya ku -50°C;
- 1,28 - ku -15-30 ° С;
- 1,27 - ku -4-15 ° С;
- 1,24-1,26 - emazingeni okushisa aphezulu.
Ngakho-ke, uma usebenzisa imoto ehlobo ezindaweni zaseMoscow noma eSt. Petersburg, ukuminyana kungaba ku-1,25-1,27 g / cm3. Ebusika, lapho amazinga okushisa ehla ngaphansi kuka-20-30°C, ukuminyana kwenyukela ku-1,28 g/cm3.
Sicela uqaphele ukuthi akudingekile ukuthi "ukhulise" ngokuzenzakalelayo. Uvele uqhubeke nokusebenzisa imoto yakho njengenjwayelo. Kodwa uma ibhethri likhishwa ngokushesha, kunengqondo ukwenza ukuxilonga futhi, uma kunesidingo, ukuyibeka ngokukhokhiswa. Uma kwenzeka imoto ime isikhathi eside emakhazeni ngaphandle komsebenzi, kungcono ukususa ibhethri futhi uyiyise endaweni efudumele, ngaphandle kwalokho izovele ikhishwe isikhathi eside sokungenzi lutho, futhi i-electrolyte izoqala cwebezela.
Amathiphu asebenzayo okusebenza kwebhethri
Umthetho oyisisekelo okufanele ukhunjulwe ukuthi akufanele kuthelwe i-sulfuric acid ebhethrini. Ukwandisa ukuminyana ngale ndlela kuyingozi, ngoba ngokwanda, izinqubo zamakhemikhali ziyasebenza, okungukuthi i-sulfation kanye nokugqwala, futhi ngemva konyaka amapuleti azoba nokugqwala ngokuphelele.
Hlola njalo izinga le-electrolyte futhi ugcwalise ngamanzi acwecwe uma lehla. Ngemuva kwalokho ibhethri kufanele lifakwe amandla ukuze i-asidi ihlangane namanzi, noma ibhethri lishajwe ejeneretha ngesikhathi sohambo olude.
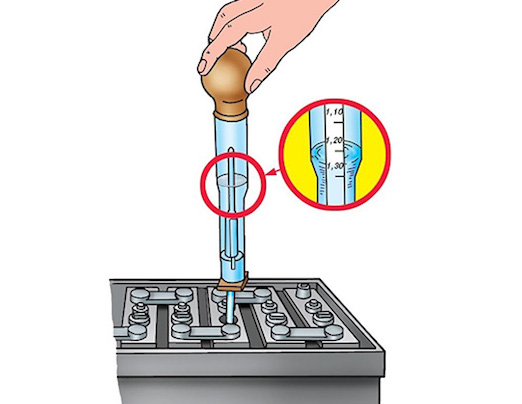
Uma ubeka imoto "ihlaya", okungukuthi, ungayisebenzisi isikhathi esithile, khona-ke, ngisho noma izinga lokushisa elivamile lansuku zonke liwela ngaphansi kwe-zero, udinga ukuqinisekisa ukuthi ibhethri ishajwe ngokugcwele. Lokhu kunciphisa ubungozi bokuqhwaza kwe-electrolyte kanye nokucekelwa phansi kwamapuleti omthofu.
Ngokuncipha kwabantu be-electrolyte, ukumelana kwayo kuyanda, okwenza kube nzima ukuqala injini. Ngakho-ke, ngaphambi kokuqala injini, fudumeza i-electrolyte ngokukhanyisa izibani zangaphambili noma ezinye izinto zikagesi isikhashana. Ungakhohlwa ukuhlola isimo samatheminali futhi uwahlanze. Ngenxa yokuxhumana okungekuhle, isiqalo akwanele ukukhiqiza itorque edingekayo.


Buka le vidiyo ku-YouTube
Iyalayisha...